What Part of the Basilar Membrane Is Sensitive to Low Frequencies
| Basilar membrane. | |
|---|---|
 Department through organ of corti, showing basilar membrane | |
 Cross department of the cochlea. | |
| Details | |
| Identifiers | |
| Latin | membrana basilaris ductus cochlearis |
| MeSH | D001489 |
| Anatomical terminology [edit on Wikidata] | |
The basilar membrane is a stiff structural element within the cochlea of the inner ear which separates two liquid-filled tubes that run along the whorl of the cochlea, the scala media and the scala tympani. The basilar membrane moves up and down in response to incoming sound waves, which are converted to traveling waves on the basilar membrane.
Structure [edit]
The basilar membrane is a pseudo-resonant structure[1] that, similar the strings on an musical instrument, varies in width and stiffness. But dissimilar the parallel strings of a guitar, the basilar membrane is a single structure with different width, stiffness, mass, damping, and duct dimensions at unlike points along its length. The move of the basilar membrane is generally described as a traveling wave.[ii] The properties of the membrane at a given point along its length decide its feature frequency (CF), the frequency at which it is most sensitive to sound vibrations. The basilar membrane is widest (0.42–0.65 mm) and least strong at the noon of the cochlea, and narrowest (0.08–0.xvi mm) and stiffest at the base (about the round and oval windows).[3] Loftier-frequency sounds localize near the base of the cochlea, while low-frequency sounds localize near the apex.
Office [edit]

Sinusoidal bulldoze through the oval window (height) causes a traveling wave of fluid–membrane motion. A modeled snapshot of fluid streamlines is shown. The wavelength is long compared to the duct height nearly the base, in what is called the long-moving ridge region, and short (0.v to 1.0 mm in typical observations[4] [5]) nearly the identify where the displacement and velocity are maximized, only before cutoff, in the brusque-wave region.
Endolymph/perilymph separation [edit]
Along with the vestibular membrane, several tissues held by the basilar membrane segregate the fluids of the endolymph and perilymph, such as the inner and outer sulcus cells (shown in yellow) and the reticular lamina of the organ of Corti (shown in magenta). For the organ of Corti, the basilar membrane is permeable to perilymph. Here the border between endolymph and perilymph occurs at the reticular lamina, the endolymph side of the organ of Corti.[6]
A base for the sensory cells [edit]
The basilar membrane is also the base for the hair cells. This office is present in all land vertebrates. Due to its location, the basilar membrane places the hair cells adjacent to both the endolymph and the perilymph, which is a precondition of hair cell role.
Frequency dispersion [edit]
A third, evolutionarily younger, function of the basilar membrane is strongly developed in the cochlea of near mammalian species and weakly developed in some bird species:[7] the dispersion of incoming sound waves to separate frequencies spatially. In brief, the membrane is tapered and information technology is stiffer at one end than at the other. Furthermore, sound waves travelling to the "floppier" finish of the basilar membrane have to travel through a longer fluid column than sound waves travelling to the nearer, stiffer end. Each function of the basilar membrane, together with the surrounding fluid, can therefore be idea of as a "mass-spring" system with different resonant properties: high stiffness and low mass, hence high resonant frequencies at the near (base) end, and low stiffness and high mass, hence low resonant frequencies, at the far (noon) cease.[8] This causes sound input of a certain frequency to vibrate some locations of the membrane more than other locations. The distribution of frequencies to places is called the tonotopic organization of cochlea.
Sound-driven vibrations travel as waves along this membrane, along which, in humans, lie virtually 3,500 inner hair cells spaced in a single row. Each cell is attached to a tiny triangular frame. The 'hairs' are minute processes on the end of the cell, which are very sensitive to movement. When the vibration of the membrane rocks the triangular frames, the hairs on the cells are repeatedly displaced, and that produces streams of corresponding pulses in the nerve fibers, which are transmitted to the auditory pathway.[nine] The outer pilus cells feed dorsum energy to amplify the traveling wave, by up to 65 dB at some locations.[ten] [xi] In the membrane of the outer hair cells there are motor proteins associated with the membrane. Those proteins are activated by sound-induced receptor potentials as the basilar membrane moves up and downwards. These motor proteins can amplify the movement, causing the basilar membrane to move a little bit more than, amplifying the traveling wave. Consequently, the inner pilus cells get more deportation of their cilia and move a little bit more and get more data than they would in a passive cochlea.
Generating receptor potential [edit]
The movement of the basilar membrane causes hair cell stereocilia motion. The hair cells are fastened to the basilar membrane, and with the moving of the basilar membrane, the tectorial membrane and the hair cells are too moving, with the stereocilia bending with the relative move of the tectorial membrane. This can cause opening and closing of the mechanically gated potassium channels on the cilia of the pilus cell. The cilia of the hair cell are in the endolymph. Unlike the normal cellular solution, depression concentration of potassium and high of sodium, the endolymph is loftier concentration of potassium and depression of sodium. And it is isolated, which means it does non take a resting potential of −70mV comparison with other normal cells, but rather maintains a potential about +80mV. Nevertheless, the base of the hair jail cell is in the perilymph, with a 0 mV potential. This leads to the pilus jail cell have a resting potential of -45 mV. As the basilar membrane moves upward, the cilia move in the direction causing opening of the mechanically gated potassium aqueduct. The influx of potassium ions leads to depolarization. On the contrary, the cilia move the other manner as the basilar membrane moves downwardly, closing more mechanically gated potassium channels and leading to hyperpolarization. Depolarization will open up the voltage gated calcium channel, releasing neurotransmitter (glutamate) at the nerve catastrophe, acting on the spiral ganglion cell, the primary auditory neurons, making them more likely to spike. Hyperpolarization causes less calcium influx, thus less neurotransmitter release, and a reduced probability of spiral ganglion prison cell spiking.
Additional images [edit]
-

Diagrammatic longitudinal section of the cochlea.
-
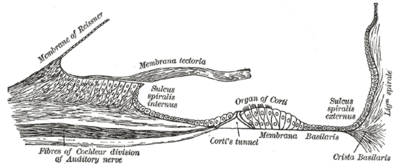
Flooring of cochlear duct.
-

Screw limbus and basilar membrane.
-

Section through the screw organ of Corti (magnified)
See likewise [edit]
Deiters cells
References [edit]
- ^ Holmes M, Cole JS (1983). "Pseudoresonance in the cochlea". In de Boer E, Viergever MA (eds.). Mechanics of Hearing. Delft: Proceedings of the IUTAM/ICA Symposium. pp. 45–52.
- ^ Fay RR, Popper AN, Bacon SP (2004). Compression: From Cochlea to Cochlear Implants. Springer. ISBN0-387-00496-3.
- ^ Oghalai JS (Oct 2004). "The cochlear amplifier: augmentation of the traveling wave within the inner ear". Current Opinion in Otolaryngology & Caput and Neck Surgery. 12 (5): 431–8. doi:ten.1097/01.moo.0000134449.05454.82. PMC1315292. PMID 15377957.
- ^ Shera CA (November 2007). "Laser distension with a twist: traveling-wave propagation and gain functions from throughout the cochlea". The Periodical of the Acoustical Society of America. 122 (5): 2738–58. Bibcode:2007ASAJ..122.2738S. doi:x.1121/1.2783205. PMID 18189566. Archived from the original on 3 July 2013.
- ^ Robles 50, Ruggero MA (July 2001). "Mechanics of the mammalian cochlea". Physiological Reviews. 81 (3): 1305–52. doi:10.1152/physrev.2001.81.iii.1305. PMC3590856. PMID 11427697.
- ^ Salt AN, Konishi T (1986). "The cochlear fluids: Perilymph and endolymph.". In Altschuler RA, Hoffman DW, Bobbin RP (eds.). Neurobiology of Hearing: The Cochlea. New York: Raven Printing. pp. 109–122.
- ^ Fritzsch B: The water-to-country transition: Evolution of the tetrapod basilar papilla; middle ear, and auditory nuclei. In: Webster DB, Fay RA, Popper AN, eds. (1992). The Evolutionary biology of hearing. Berlin: Springer-Verlag. pp. 351–375. ISBN0-387-97588-8.
- ^ Schnupp J, Nelken I, King A (2011). Auditory Neuroscience. Cambridge MA: MIT Press. ISBN978-0-262-11318-2.
- ^ Beament J (2001). "How We Hear Music: the Relationship Betwixt Music and the Hearing Machinery". Woodbridge: Boydell Press: 97.
- ^ Nilsen KE, Russell IJ (July 1999). "Timing of cochlear feedback: spatial and temporal representation of a tone across the basilar membrane". Nature Neuroscience. 2 (7): 642–8. doi:10.1038/10197. PMID 10404197. S2CID 2380374.
- ^ Nilsen KE, Russell IJ (October 2000). "The spatial and temporal representation of a tone on the guinea pig basilar membrane". Proceedings of the National University of Sciences of the United states of america of America. 97 (22): 11751–8. Bibcode:2000PNAS...9711751N. doi:10.1073/pnas.97.22.11751. PMC34345. PMID 11050205.
External links [edit]
- Auditory Neuroscience | The Ear several animations showing basilar membrane motility under various stimulus conditions
- Functional beefcake of the inner ear: enough of images, animations, and very curtailed functional explanations
- Basilar Membrane Simulator Video and Scripts to Simulate the Basilar Membrane
- The function of the basilar membrane in sound reception: practiced explanation and diagrams
Source: https://en.wikipedia.org/wiki/Basilar_membrane#:~:text=High%2Dfrequency%20sounds%20localize%20near,sounds%20localize%20near%20the%20apex.
0 Response to "What Part of the Basilar Membrane Is Sensitive to Low Frequencies"
Post a Comment